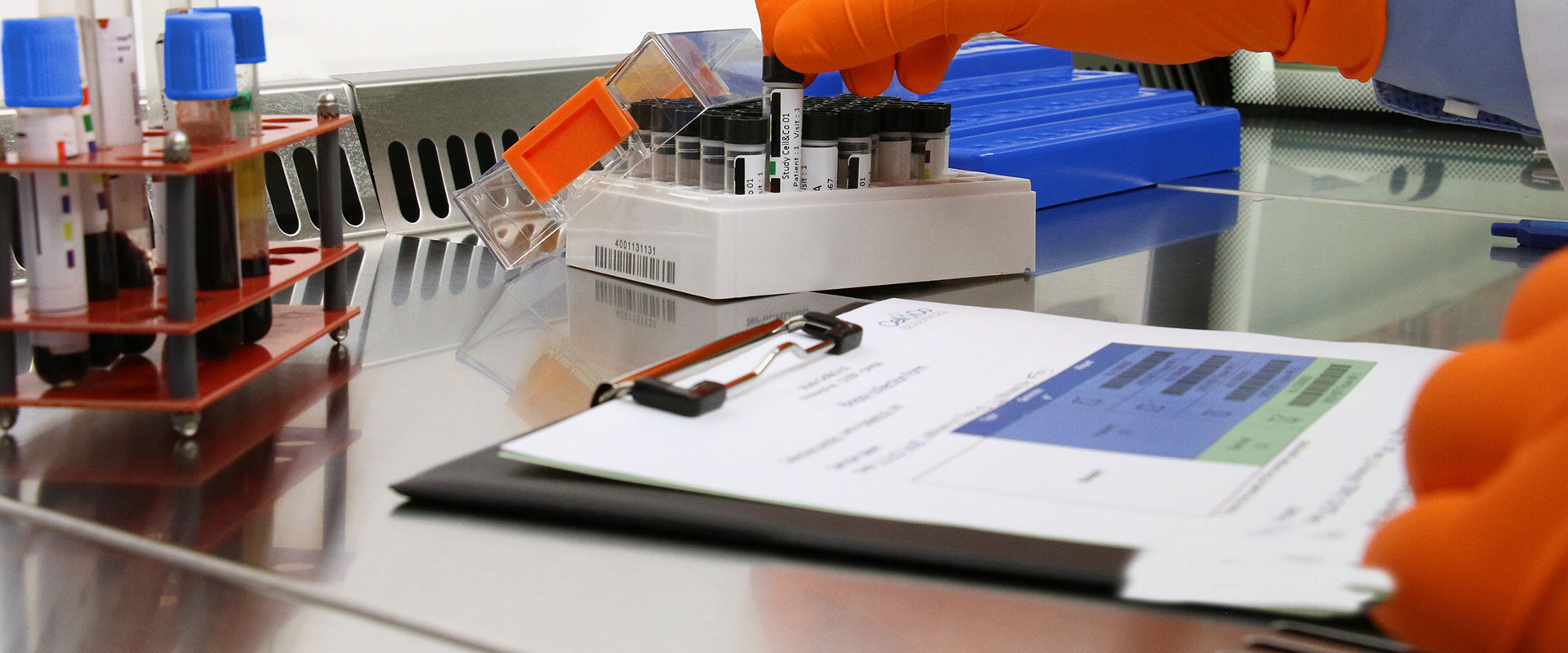
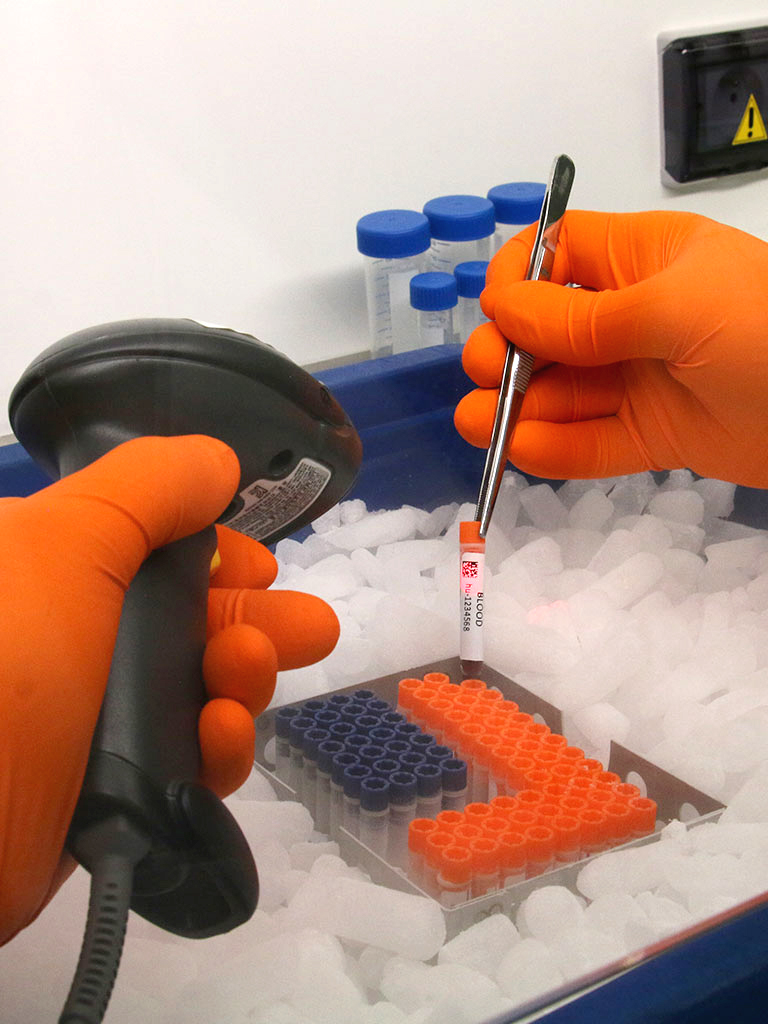
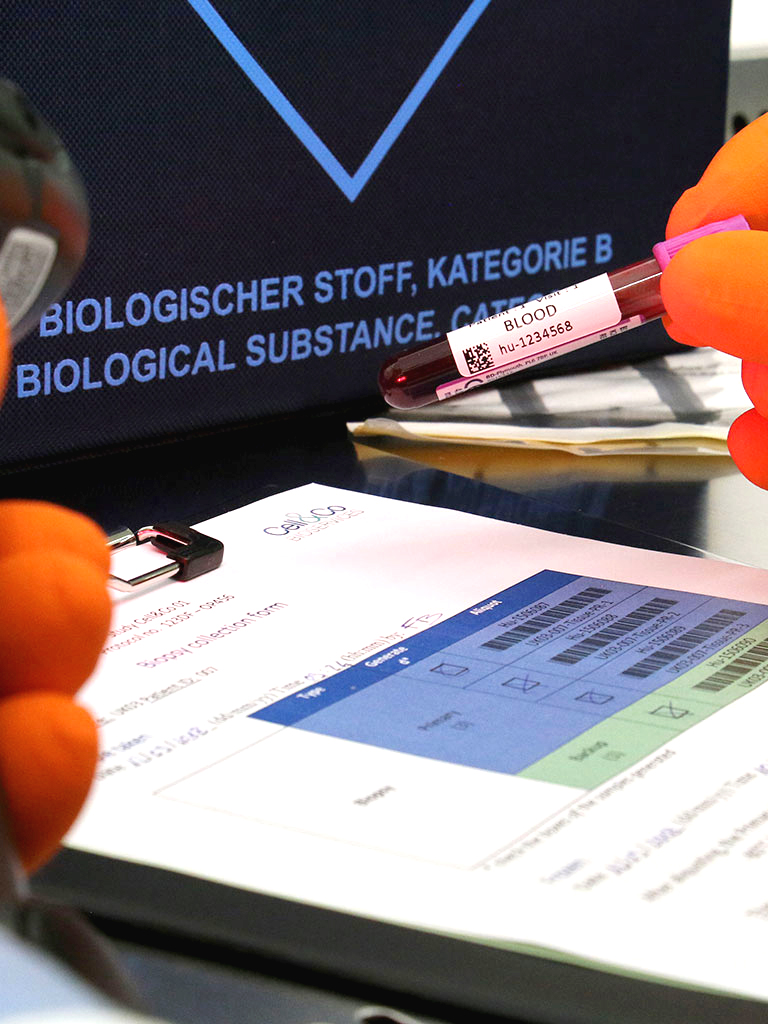
We were setting up a clinical study in DRC (Democratic Republic of Congo) where we were going to assay thyroid hormones. As sample storage and transportation conditions were planned for remote locations, we had to cover the risk of the cold chain being broken and be able to prove in advance that our analytical data would be reliable should the samples thaw. Our complex issue was explained to Cell&Co, which was able to rise to the challenge by suggesting a bespoke stability test for thyroid hormones. We gained immense support from their commitment and advice and it is clear that they were able to fully understand our issues. Their adaptability and empathy subsequently made the collaboration uncomplicated and highly successful. Lastly, the quality of the study's stability results enabled us to carry out our clinical trial with complete peace of mind.
DNDI
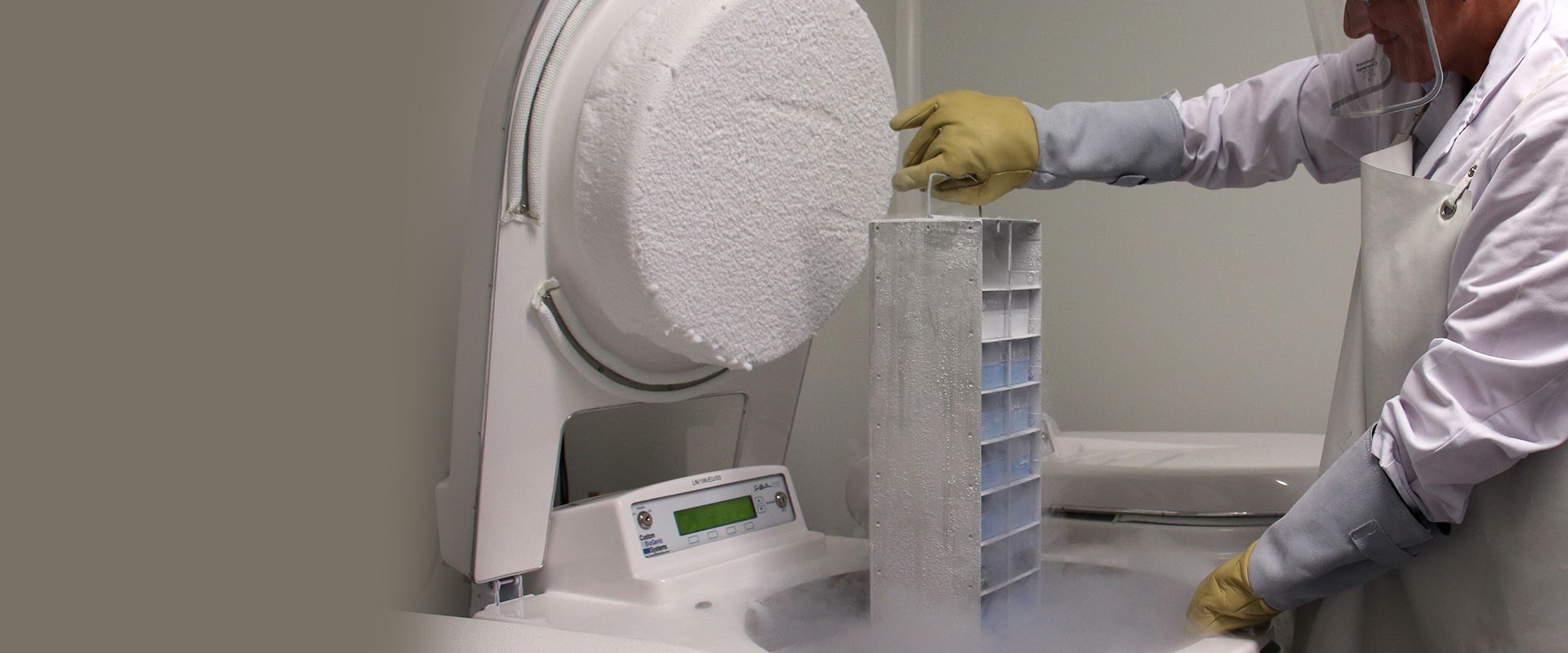
Our tissue grafts are stored at -80°C. Notwithstanding our emergency systems in the event of our storage units failing, we decided to look for another backup system to provide secure infrastructure and a rapid response around the clock. Cell&Co met these criteria and their successfully delivered services fully support our decision. Use of the Cell&Co emergency storage solution has been approved by our supervisory and regulatory authority. We are therefore able to save a large number of our high value-added products in the event of an incident. This partnership also removes the need to invest in additional equipment, or even new premises.
Anonymous (Tissue bank for therapeutic use)
Having explained our needs and technical constraints (sample types and quantities, pre-analytical conditions, etc.), through the different projects entrusted to it Cell&Co has been able to demonstrate its ability to create appropriate specifications and ingenious equipment able to facilitate our work and the work performed at investigator sites. We also particularly appreciate the quality and speed of their responses throughout the study cycle, and their transparency and proactivity, both in terms of project management and risk management. Cell&Co therefore meets our objective of establishing lasting relationships based on trust with all our partners.
ONXEO
The search for biomarkers is a growth area in clinical trials and generates large volumes of samples. As we were looking to safeguard and centralize these resources within a high-quality biobank, we conducted a full audit of Cell&Co before selecting this partner. Cell&Co arranged for central collection of all our samples from over 70 French hospitals. Our collaboration has continued by extending to sample collection and logistics for major multicentre clinical studies. We particularly appreciate the team’s responsiveness and professionalism.
IFCT (French Cooperative Thoracic Intergroup)
Cell&Co were recommended by one of our trusted service providers to provide GMP-compliant, secure, monitored facilities which would be able to store 50% of our cell banks under strict temperature conditions. Following a vendor audit, Cell&Co was selected for this service, which also included the provision of all regulatory documentation required for the import and storage of the materials, organising the logistics for the transfer and receipt of the materials under GMP conditions. The contractual process and high quality service were provided by Cell&Co’s friendly and responsive staff.
SCANCELL
We contracted Cell&Co to store, at a single, secure site, all the remaining samples after closeout of our clinical studies. Cell&Co assembled a large number of samples from European CROs into a single collection. The objective was to have easy access to these biological resources in order to be able to repeat assays or perform new analyses. Cell&Co’s understanding, flexibility and responsiveness enable them to easily accommodate our new requests. This partner boosts the efficiency of our organization and simplifies our work, enabling us to focus on our core business.
IPSEN
Cell&Co is a specialized service provider that impressed us by the quality of its infrastructure, its ambition and its wide range of solutions. We have benefited from the team’s expertise and advice in setting up the group’s single biobank, covering our substantial livestock operation. Cell&Co’s client relations and the state of-the-art tools it proposes have been genuine assets in this strategic project that is far removed from our core business. Cell&Co has become our trusted partner for long-term sample storage.
Royal Canin